Plant 1
Plant 1
 This is located at 35, 43 & 44 HPSIDC Industrial Area Baddi (H.P.). This
is a WHO - GMP Certified Plant with a total area of 6600 square meters.
This plant has the following Units & Departments:
This is located at 35, 43 & 44 HPSIDC Industrial Area Baddi (H.P.). This
is a WHO - GMP Certified Plant with a total area of 6600 square meters.
This plant has the following Units & Departments:
A. Formulation Units
B. Engineering and Maintenance Department
C. Admin Department
D. Store Department
E. Quality Control Department
F. Quality Assurance Department
G. Stability & Formulation Development Department
H. Certification
This Unit comprises of 10 Formulation Production Sections, these are-
1. Tablet Section
2. Capsules Section
3. Dry Syrup Section
4. Liquid Orals Section
5. Topical Section-Cream/ Ointment / Lotions
6. External/Dusting Powder Section
7. Powder/Granules Section
8. Beta Lactam Tablet Section
9. Beta Lactam Capsules Section
10. Beta Lactam Dry Syrup Section
 A1. TABLETS SECTION:
A1. TABLETS SECTION:
• Having Capacity of Approx 45 Crores Tablets per Month.
• The tablets section consists of 4 sub sections namely.
 A1.1 Granulation Section -
A1.1 Granulation Section -
• This section consists of high technology equipment like
Rapid Mass Mixer (RMG), Fluid Bed Dryer, Paste Kettle ,
Vibro Sifter, Octagonal Blender, etc.
• Its Working Team consists of Approximately 10 Chemists with 1 Executive
& 1 Senior Executive.
• Additionally the team has more than 10 Operators & Senior Operators.
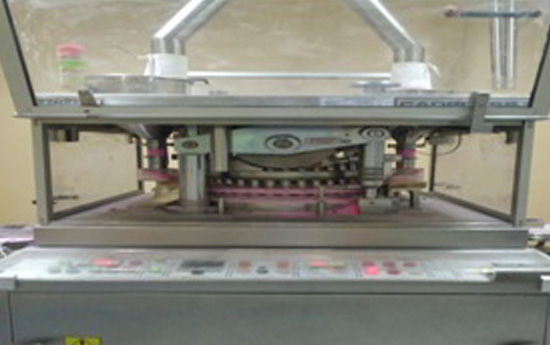
 A1.2 Compression Section -
A1.2 Compression Section -
• This section consists of state of the art
compression machines like
2 Bilayer Compression Machine, 16 Compression
Machines of Cadmach.
• Its Working Team consists of approximately 10
Chemists, with 1 Executive, 1 Senior Executive and 10
Operators & Senior Operators led by assistant manager
production.
 A1.3 Coating Section-
A1.3 Coating Section-
• This section consists of fully HVAC rooms, 3 semi
automatic Coating machines and 2 fully Automatic
coating machines for Coating of tablets
• Its Working Team consists of 2 Chemists, 1 Executive,
1 Senior Executive and 5 Operators & Senior
Operators.
A1.4 Packing Section -
• Pharmaceutical packaging (or drug packaging) is the
process for pharmaceutical preparations which  involves packaging of drug dosage form in a suitable
container which ensures safe travel of drug from
manufacturing company to patients through drug
distribution channels .
involves packaging of drug dosage form in a suitable
container which ensures safe travel of drug from
manufacturing company to patients through drug
distribution channels .
• This section consists of fully air conditioned 15 Packing
Lines.
• It consists of 10 Blister packing machines, 3 Alu-Alu blister packing
Machines & 2 Aluminium strip Machines .
• Its Working Team consists of 7 Chemists, 1 Executive, 1 Senior Executive
and 16 Operators & Senior Operators.
• Loose Tablet Packing in pouches is also done by two automatic form fill
and seal machines.
 A2. CAPSULES SECTION:
A2. CAPSULES SECTION:
• Manufacturing of Capsules is a special pharmaceuticals unit operation in
WINGS with controlled atmosphere fitted with
HVAC and dehumidifiers for control of temperature
and relative humidity during capsules production
for safety and stability of active drugs in capsules.
• A number of modified techniques are used for production of capsules.
• Capsules section consists of two fully settled capsules production
sections having fully automatic and 4 SA-09 semi automatic capsule filling machines and
other accessory machines for filling of different sizes on capsules.
• Having Capacity of Approx 4.5 Crores Capsules per Month.
• Its Working Team consists of 2 Chemists with 1 Asst Manager and 5
Operators & Senior Operators and Workers.
 A3. LIQUID SECTION:
A3. LIQUID SECTION:
• This section consists of two fully automatic liquid
manufacturing and 4 fully automatic Liquid filling
Lines, with capacity of 27 Lakhs Bottles per Month .
• Its Working Team consists of 1 Asst Manager, 4
production chemists and 8 Operators & Senior
Operators and Workers.
A4. TOPICAL SECTION : (Cream/ Ointment / Lotion)
• An oil-based preparation that is applied to the skin.  Whereas an ointment has an oil base, a cream is
oil in water emulsion .
Whereas an ointment has an oil base, a cream is
oil in water emulsion .
• This section has fully automatic two Ointment
Manufacturing plants with accessory machines
where Ointments,Creams & Lotions are prepared
with capacity of 45 Lakhs Tubes /Month.
• Its Working Team consists of 1 assistant production manager , 1 Senior
Executive, 4 Chemists, 5 Operators & Senior Operators and Workers.
A5. EXTERNAL POWER SECTION :
(like Clotrimazole Dusting Powder.)

A6. POWDER SECTION:
(like ORS & Sachets)
• We are having Automatic Machine for filling powders in
pouches
• We are also having automatic powder filling machines for
ORS packaging ,sachet packaging from 1 gm to 50 gms.
A7. BETA LACTAM ANTIBIOTICS SECTION: (like Tablets, Capsules and Dry Syrups)
• Dedicated facility adhering to the latest segregation regulations- GMP
Standards.
• Have a separate block , separate infrastructure , separate entrance and
separate production team dedicated for beta lactam products .
• This section is further classified for Beta Lactum Tablets, Capsules & Dry
Syrups .
A7.1 BETA LACTAM TABLETS:-

A7.2 BETA LACTAM CAPSULES:-
A7.3 BETA LACTAM DRY SYRUP:-

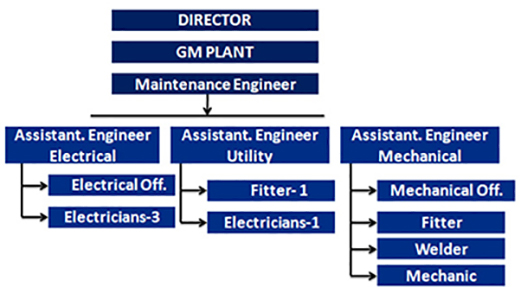
 B.1 Plants Engineering/ Maintenance is well
supported with
B.1 Plants Engineering/ Maintenance is well
supported with
1. HT Supply 1100 volts
2. HT Transformers capacity 1200 KVA
3. DG sets capacity (480kva , 180 kVA X3 )
4. Air compressors 180 CFM, 100CFM AND 60CFM
oil less screw compressors for oil free air.
5. Chilling plant for air conditioning of production
sections
6. 2 Steam Boilers 600KG/hour
7. RO Water PlantX2
8. DM Water Plant X2
9. DM water supply system
10. Cooling Towers
11. Fire extinguisher and water hydrant system
12. Effluent Treatment Plant (ETP)
13. Rainwater Harvesting System
 1. Admin department is the backbone of an organization.
1. Admin department is the backbone of an organization.
2. An effective administrator is an asset to an
organization.
3. Provides administrative and technical support to
various departments-.human resources (HR),
budgetary, strategic planning, legal affairs, facilities
and security.
4. Ensures the smooth flow of information from one part
to the other.
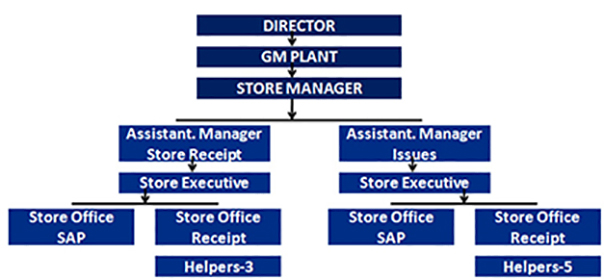
 1. This is concerned with quantity or supply of RM, PM & General Utility Materials are kept for use as needed.
1. This is concerned with quantity or supply of RM, PM & General Utility Materials are kept for use as needed.
2. The plant has a well established Store Department with sufficient space for raw material & Packing Material.
 1. The Quality Control Department is responsible
for quality control of all materials , starting
from raw material to packing material ,
environment and finished goods .
1. The Quality Control Department is responsible
for quality control of all materials , starting
from raw material to packing material ,
environment and finished goods .
2. At Wings we are having a well furnished
Quality Control Lab. Wings Biotech QC Deptt is
duly certified by HP Drug Department as
Observing Good Lab Practices (GLP certificate) which consists of the
following sub sections.
A. Raw Material Analysis
B. Finished Goods Analysis
C. Microbiology Section
D. Packing Material analysis
E. Stability Section
F. Control sample Section
G. Instrumental Analysis section
H. Wet/ Experimental Lab
I. Formulation & development Lab. for Tablets, Capsules, Liquid Orals & Tropical Preparations like Ointment, Cream & Lotions Oint.
1. The QC Department team consists of 25 people that has HOD,
Manager
QC, Asst Manager QC, Team Leader, Sr. Exe, Exe.Chemist, Trainee
Chemist & Lab Asst.
1. Ours Labs are equipped with the latest state-of-the- art Equipment .
a. Instrument Labs:

b. Chemical /Raw Material (RM) Testing Labs.:

c. Instrumentation/Finished Goods (FG) Testing Labs:

d. Micro Labs.:

e. Packaging Material (PM)Testing Labs:

f. Wet/Experimental Labs:

g. Stability Labs:

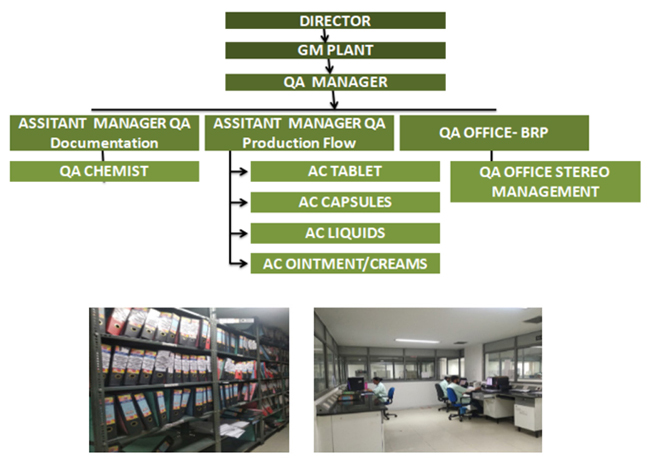
 1. Quality Assurance Department is a way of
preventing mistakes and defects in manufactured
products and avoiding problems when delivering
products or services to customers.
1. Quality Assurance Department is a way of
preventing mistakes and defects in manufactured
products and avoiding problems when delivering
products or services to customers.
2. We are having IPQA Department which works hand
to hand with the Production Department for
assuring Quality of Products, Market Complaint
Department, Regulatory Affairs and
Documentation Department which comply GMP Record & Coordinates with
all other departments for Self Inspection Programme,Training & Various
other Records.
Our Plant is WHO-GMP certified, GMP certified and also has GLP certified for
its Laboratories, Drug License and the medicine we manufactured are also
having Drug Approval.